The eTMF software for worry-free inspections
eTMF Connect uses automation and built-in workflows to help scaling life sciences teams maintain inspection-ready documentation, start studies faster, and achieve real-time visibility into TMF completeness without the complexity of traditional eTMF systems.
Book a demo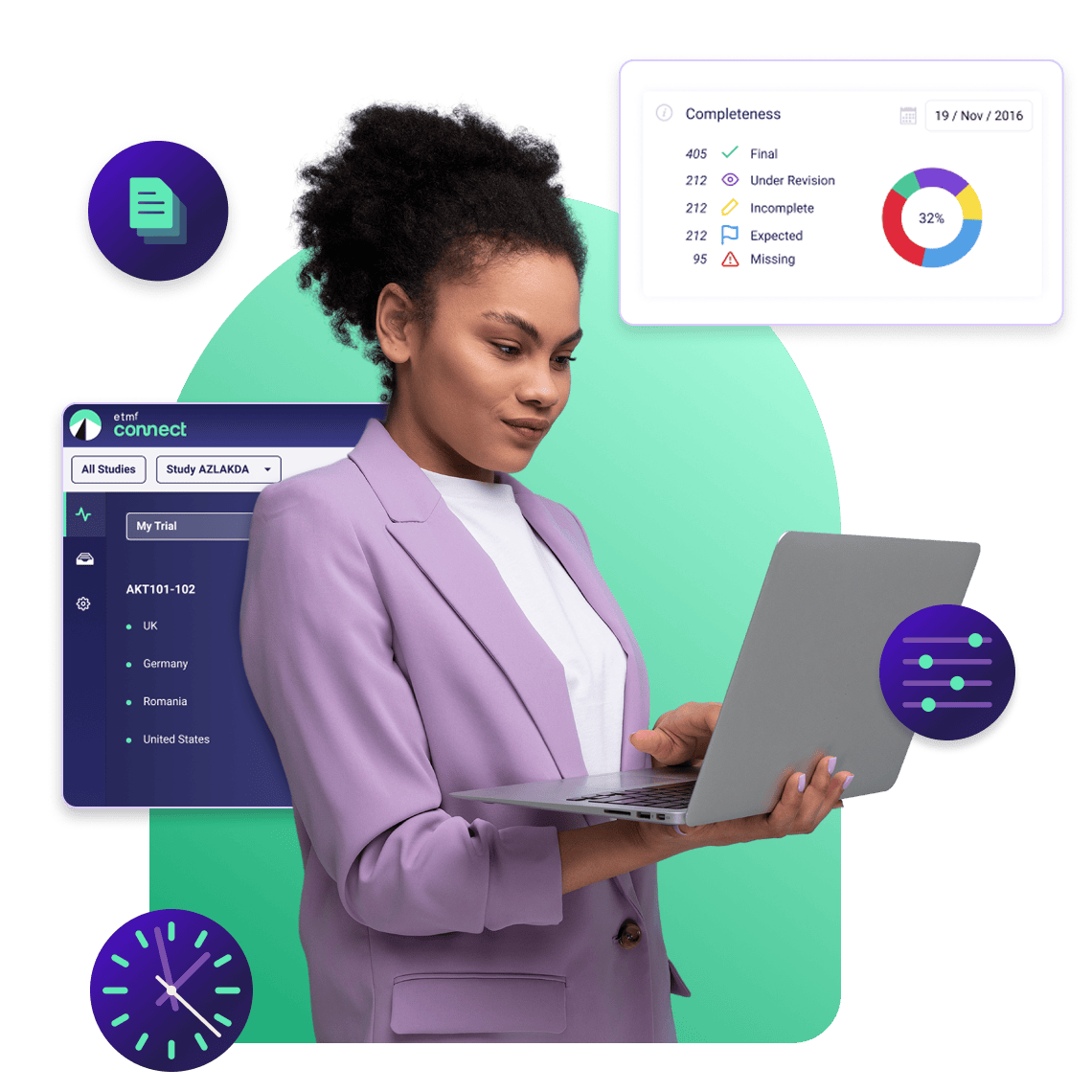




























Scale your clinical operations without scaling your workload

Pre-Configured TMF Structures
Start studies faster
Accelerate study startup with pre-configured TMF structures aligned to the TMF Reference Model, automated workflows, and reusable templates that eliminate repetitive setup work.




Inspection Readiness Monitoring
Catch issues before inspectors do
Get real-time visibility into TMF completeness, missing documents, and quality metrics through the eTMF Navigator, so you're always prepared for regulatory inspections.




Distributed Team Collaboration
Empower remote collaboration
Enable sites, CROs, and internal teams to contribute documentation seamlessly through email-to-TMF capabilities, and role-based access, supporting decentralized and hybrid trials.



Pre-Configured TMF Structures
Start studies faster
Accelerate study startup with pre-configured TMF structures aligned to the TMF Reference Model, automated workflows, and reusable templates that eliminate repetitive setup work.




Inspection Readiness Monitoring
Catch issues before inspectors do
Get real-time visibility into TMF completeness, missing documents, and quality metrics through the eTMF Navigator, so you're always prepared for regulatory inspections.




Distributed Team Collaboration
Empower a remote workforce
Enable sites, CROs, and internal teams to contribute documentation seamlessly through email-to-TMF capabilities, and role-based access, supporting decentralized and hybrid trials.





What sets Montrium apart
See what needs attention instantly
The eTMF Navigator provides a visual overview of TMF completeness at the study, country, and site levels. Identify missing documents immediately and drag and drop files directly onto placeholders to update completeness in real-time.
Eliminate filing bottlenecks
Sites can email documents directly to study-specific inboxes where they're queued for indexing. Auto-filing based on document naming conventions means your team spends less time on administrative tasks and more time on critical study activities.

Empower remote collaboration
Real-time collaborative authoring, configurable workflows ensure all stakeholders can contribute effectively, whether they're at headquarters, global sites, or conducting remote visits. Maintain full compliance and control while enabling seamless collaboration.
Work with industry-leading experts
Our team preps organizations for inspections, builds GCP-compliant frameworks, and helps define industry TMF standards. docGet guidance from people who know what inspectors look for, not generic support.

Results that speak for themselves

eTMF Connect has been worth the financial investment, we have become more efficient with our staff resources and maintaining the TMF, and can take on new projects without adding significant time and costs.



achieved an estimated increase in trial documentation efficiency of



Security & compliance
Montrium protects customer data through rigorous security measures and compliance practices. All personnel with access to sensitive data are thoroughly vetted, maintain strict confidentiality, and receive ongoing security training. Testing protocols include penetration testing, vulnerability scanning, and code analysis. Cloud environments leverage Microsoft Azure's security features with data isolation, encryption in transit and at rest, and continuous monitoring aligned with SOC 2 standards.

FDA 21 CFR Part 11
Montrium systems meet FDA 21 CFR Part 11 requirements through secure user access, comprehensive audit trails, and electronic signatures that are uniquely assigned, securely linked to records, and protected against unauthorized use.

EudraLex Volume 4 Annex 11
Montrium products align with Annex 11 principles for computerized systems in GMP-regulated environments. Solutions support data integrity standards, provide secure audit trails, and enforce strict access controls through a risk-based validation approach.

SOC 2® Type I
Montrium successfully completed the AICPA SOC 2 Type I audit, confirming our information security practices meet SOC 2 standards for security.

Audit support
Montrium facilitates vendor qualification audits through transparent access to documentation and compliance evidence. We also support customers during regulatory inspections by supplying compliance documentation and responding promptly to inquiries.

ISO 9001:2015 QMS Alignment
Montrium's Quality Manual and procedural documents satisfy ISO 9001:2015 requirements for quality documentation, policy, and objectives.

GDPR
Montrium has implemented appropriate technical and security processes to ensure GDPR compliance.
“eTMF Connect is a huge improvement. We went from one study to three studies, from one country to several, thereby more than tripling the number of clinical sites. It would not have been possible to scale the way we needed to without eTMF Connect.”


.webp)
Ready to transform your TMF management?
See how eTMF Connect can help your team start studies faster, maintain inspection readiness, and scale clinical operations efficiently.
 Schedule a demo
Schedule a demoFrequently asked questions
Find answers to common questions about eTMF Connect and its functionalities.
.webp)
Resources for your TMF team

Building a Risk-based TMF Management Framework
In this first-of-its-kind white paper, Montrium's experts detail their new methodology for risk-based TMF management.

CFO Pitch Guide
The CFO Pitch Guide is your playbook for executive conversations. Learn how to translate clinical tech benefits into CFO-ready messaging that drives clarity, confidence, and buy-in.

TMF Toolkit
This TMF Toolkit is a curated collection of useful links, tools and resources compiled by TMF subject matter experts to support the TMF community. Bookmark it and check back often for new content and updates.

